Biochemical composition and antioxidant properties of some seaweeds from Red Sea coast, Egypt
Abstract
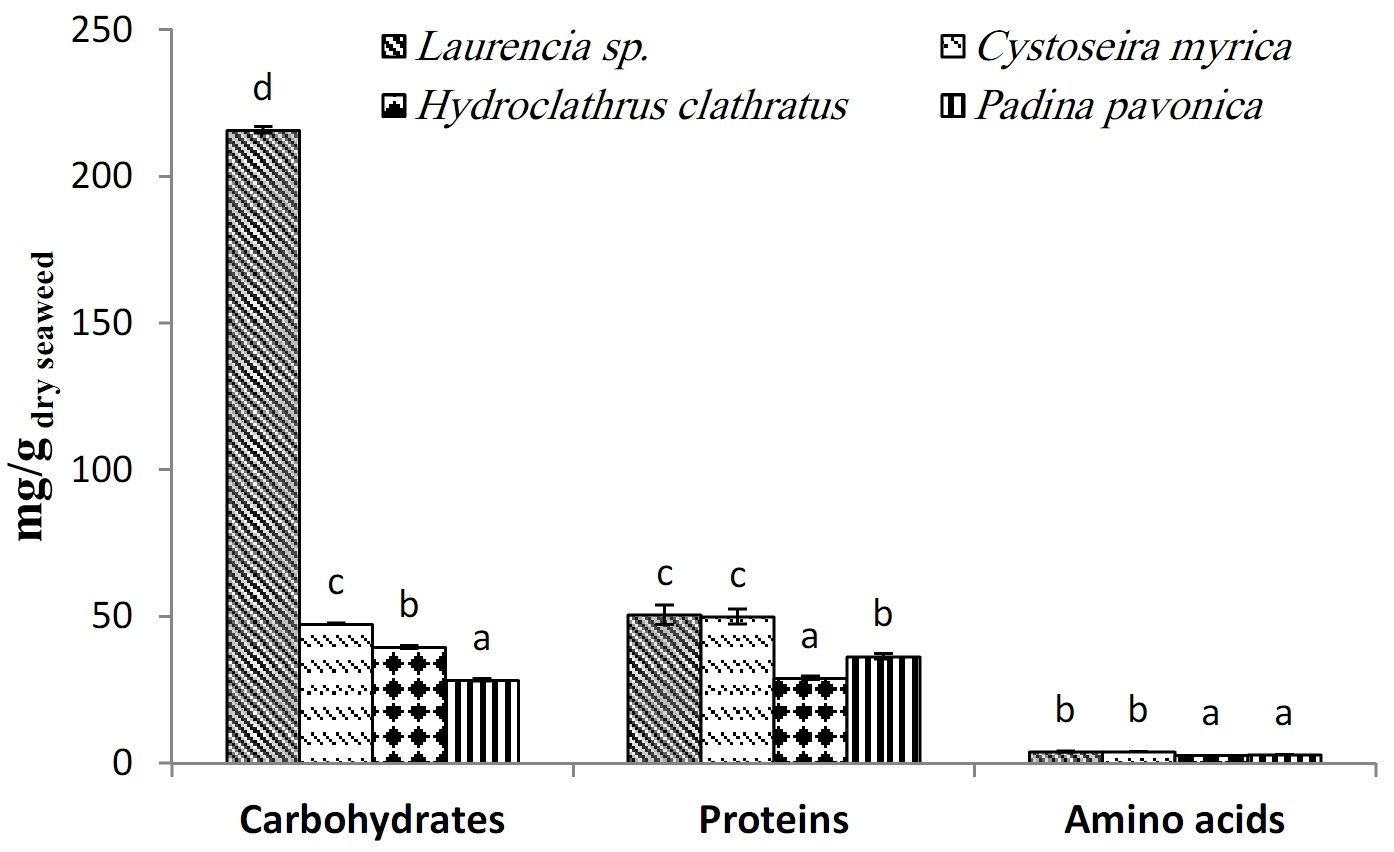
The current study investigated the biochemical composition and antioxidant properties of four seaweeds: Laurencia sp. (Rhodophyta), Cystoseira myrica, Hydroclathrus clathratus and Padina pavonica (Ochrophyta). The highest amount of carbohydrates was (215.78 mg/g dry wt.) in Laurencia sp. and proteins content was maximum (50 mg/g dry wt.) in Laurencia sp. and Cystoseira myrica. The highest values of free amino acid content were recorded in the brown seaweed species Cystoseira myrica (4.01 mg/g dry wt.). The pressurized hot water extract of Cystoseira myrica has the highest total phenolic content (1.61 mg GAE/g dry wt.). Cystoseira myrica contained the highest amounts of flavonoids (3.35 mg/g dry wt.), ascorbic acid (9.07 mg/g dry wt.) and α-tocopherol (27.25±0.00 abs. at 520 nm/g dry wt.). Furthermore, the ethyl alcohol extract of Cystoseira myrica showed high antioxidant capacities (541.6 mg/g dry wt.) and achieved the most powerful reducing ability among all of the different extracts of algal species. Statistical evaluation by Spearman correlation between the TAC assay and the total phenolic contents was found to be significant, but the correlation was nonsignificant between FRAP assay and the total phenolic contents. The composition of elements of the studied seaweed species was also analyzed. The most significant macro-elements present in the studied seaweeds were K, Na and Ca, representing that the seaweeds are good sources of these elements. Since, these seaweeds are widespread in the Egyptian waters, their biochemical composition and antioxidant capacities made them promising candidates for industrial, nutritional and pharmaceutical applications.
Downloads
References
2. Jeeva S, Marimuthu J, Domettila C, Anantham B, Mahesh M. Preliminary phytochemical studies on some selected seaweeds from Gulf of Mannar, India. Asian Pac J Trop Biomed. 2012; 2(1): 30-33.
3. Polat S, Ozogul Y. Seasonal proximate and fatty acid variations of some seaweeds from the northeastern Mediterranean coast. Oceanologia. 2013; 55(2): 375-391.
4. Goo BG, Baek G, Jin Choi D, Il Park Y, Synytsya A, Bleha R, et al. Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Biores Technol. 2013; 129: 343-350.
5. Kurniawati HA, Ismadji S, Liu JC. Microalgae harvesting by flotation using natural saponin and chitosan. Biores Technol. 2014; 166: 429-434.
6. Pérez MJ, Falqué E, Domnguez H. Antimicrobial action of compounds from marine seaweed. Mar Drugs. 2016; 14(3): 52.
7. Holdt SL, Kraan S. Bioactive compounds in seaweeds: functional food applications and legislation. J Appl Phycol. 2011; 23: 543-597.
8. Mak W, Hamid N, Liu T, Lu J, White WL. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidantactivities. Carb Polym. 2013; 95: 606-614.
9. Mak W, Wang SK, Liu T, Hamid N, Li Y, Lu J, et al. Antiproliferation potential and content of fucoidan extracted from sporophyll of New Zealand Undaria pinnatifida. Front Nutr. 2014; 1: 1-10.
10. Zhou AY, Robertson J, Hamid N, Ma Q, Lu J. Changes in total nitrogen and amino acid composition of New Zealand Undaria pinnatifida with growth, location and plant parts. Food Chem. 2015; 186: 319-325.
11. Rodriguez EB, Rodriguez-Amaya DB. Formation of apocarotenals and epoxycarotenoids from β-carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chem. 2007; 101(2): 563-572.
12. Yuan YV, Carrington MF, Walsh NA. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem Toxicol. 2005; 43: 1073-1081.
13. Chew YL, Lim YY, Omar M, Khoo, KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT. 2008; 41: 1067-1072.
14. Fernando IS, Kim M, Son KT, Jeong Y, Jeon YJ. Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J Med Food. 2016; 19(7): 615-628.
15. Plaza M, Cifuentes A, Ibáñez E. In the search of new functional food ingredients from algae. Trends Food Sci Technol. 2008; 19: 31-39.
16. Aruoma IO. Antioxidant action of plant foods. Use of oxidative DNA damage, as a tool for studying antioxidant efficacy. Free Radical Res. 1999; 30: 419-427.
17. Lordan S, Ross RP, Stanton C. Marine bioactives and functional food ingredients: potential to reduce incidence of chronic diseases. Mar Drugs. 2011; 9: 1056-1100.
18. Dhargalkar VK. Uses of seaweeds in the Indian diet for sustenance and well-being. Sci Cult. 2014; 80: 192-202.
19. Gouda EA. Obstacles to sustainable tourism development on the Red Sea Coast. Int J Innov Educ Res. 2015; 3: 165-176.
20. Guiry MD, Guiry GM. AlgaeBase. National University of Ireland, Galway [accessed 15 August 2013]. http:// www.algaebase.org
21. Meñez EG, Mathieson AC. The marine algae of Tunisia. Smithsonian Contributions to the Marine Sciences, Washington. 1981.
22. Nizamuddin M. The green marine algae of Libya. Elga, Bern. 1991; 227 pp.
23. Aleem AA. The marine algae of Alexandria, Egypt. Alexandria. 1993.
24. Jha B, CRK, Reddy MC, Thakur MU, Rao. Seaweeds of India: the diversity and distribution of seaweeds of the Gujarat Coast (Vol. 3). Springer, New York. 2009.
25. Fales FW. The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem. 1951; 193: 113-118.
26. Schlegel HG. Die Verwertung organischer sauren duch Chlorella in licht. Planta. 1956; 47: 510-526.
27. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
28. Moore S, Stein WH. Photometric ninhydrin method for use in the ehromatography of amino acids. J Boil Chem. 1948; 176: 367-388.
29. Kofalvi SA, Nassuth A. Influence of wheat streak mosaic virus infection phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol Mol Plant Pathol. 1995; 47: 365-377.
30. Moreno MIN, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71: 109-114.
31. Jagota SK, Dani HM. A New colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem. 1982; 127: 178-182.
32. Pearson DC. The chemical analysis of food. 7th edn. Churchill Livingstone Edinburgh, London, 1976: 19-35.
33. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999; 269: 337-341.
34. Oyaizu M. Studies on product of browning effect reaction prepared from glucose amine. J Nutr. 1986; 44: 307-315.
35. Jackson ML. Soil chemical analysis. Prentice-Hall, Inc. Englewood Cliffs, N.J. New Delhi, India. 1973.
36. Page AL, Miller RH, Keeney DR. Methods of soil analysis. Part 2: Chemical and microbiological properties. 2nd edn. Am Soc Agron Inc Soil Sci Soc Am. Madison, Wisconsin, USA. 1982.
37. Munier M, Dumay J, Morançais M, Jaouen P, Fleurence J. Variation in the biochemical composition of the edible seaweed Grateloupia turuturu Yamada harvested from two sampling sites on the Brittany coast (France): The influence of storage method on the extraction of the seaweed pigment R-phycoerythrin. J Chem. 2013: ID 568548.
38. Kolanjinathan K, Ganesh P, Saranraj P. Pharmacological importance of seaweeds: a review. World J Fish Mar Sci. 2014; 6(1): 1-15.
39. Ismail GA. Biochemical composition of some Egyptian seaweeds with potent nutritive and antioxidant properties. Food Sci Technol Campinas. 2017; 37(2): 294-302.
40. Ibañez E, Cifuentes A. Benefits of using algae as natural sources of functional ingredients. J Sci Food Agricult. 2013; 93: 703-709.
41. Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol. 1999; 10(1): 25-28.
42. Plaza M, Turner C. Pressurized hot water extraction of bioactives. TrAC Trends Anal Chem. 2015; 71; 39-54.
43. Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC. Seasonal variation in the chemical composition of two tropical seaweeds. Biores Technol. 2006; 97: 2402-2406.
44. Chakraborty K, Praveen N, Vijayan KK, Rao GS. Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, sargassaceae) collected from Gulf of Mannar. Asian Pac J Trop Biomed. 2013; 3(1): 8-16.
45. Kim SM, Kang K, Jeon JS, Jho EH, Kim CY, Nho CW, Um BH. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effects against oxidative stress induced by tert-butyl hyperoxide. Appl Biochem Biotechnol. 2011; 165: 1296-1307.
46. Heo SJ, Cha SH, Lee KW, Jeon YJ. Antioxidant activities of red algae from Jeju Island. Algae. 2006; 21: 149-156.
47. Yuan YV, Bone DE, Carrington MF. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005; 91: 485-494.
48. Heo SJ, Cha SH, Lee KW, Cho SK, JeonYJ. Antioxidant activities of Chlorophyta and Phaeophyta from Jeju Island. Algae. 2005; 20: 251-260.
49. Zhang WW, Duan XJ, Huang HL, Zhang Y, Wang BG. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J Appl Phycol. 2007; 19: 97-108.
50. Sarojini Y, Lakshminarayana K, Seshagiri Rao P. Variations in distribution of flavonoids in some seaweed of Visakhapatnam coast of India. Pharma Chemica. 2012; 4(4): 1481-1484.
51. Kokilam G, Vasuki S. Biochemical and phytochemical analysis on Ulva fasciata and Caulerpa taxifolia. Int J Pharm Phar Sci Res. 2014; 4(1): 7-11.
52. Collins KG, Fitzgerald GF, Stanton C, Ross RP. Looking beyond the terrestrial: the potential of seaweed derived bioactives to treat non-communicable diseases. Mar Drugs. 2016; 14(60): 1-31.
53. Rapisarda P, Bianco ML, Pannuzzo P, Timpanaro N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Post Harv Boil Technol. 2008; 49(3): 348-354.
54. Dixon RA, Paiva NL. Stress induced phenylpropanoid metabolism. Plant Cell. 1995; 7: 1085-1097.
55. Nisizawa, K. Seaweeds Kaiso: Bountiful Harvest from the Seas. Japan Seaweeds Assoc. 2006: 106.
56. Anderson J, Young L, Long E. Potassium and Health in Food and Nutrition Series, Colorado State University, Colorado. 2008.
57. Ito K, Hori K. Seaweed: chemical composition and potential uses. Food Rev Int. 1989; 5: 101-144.
58. Khairy HM, El-Sheikh MA. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J Biol Sci. 2015; 22(5): 623-630.
59. Mabeau S, Fleurence J. Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci Technol. 1993; 4: 103-107.
60. Mišurcová L, Machů L, Orsavová J. Seaweed minerals as nutraceuticals. Adv Food Nutr Res. 2011; 64: 371-390.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




