Synthesis of nicotine derivatives and evaluation of their anti-bacterial activity
Abstract
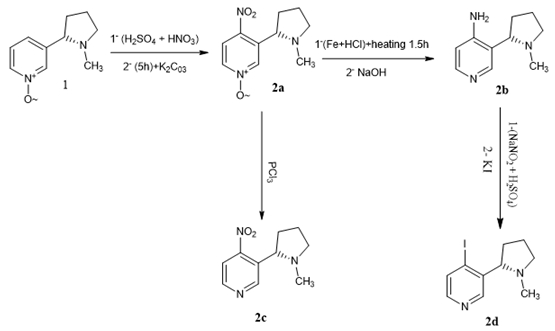
Using a convergent synthetic method, a series of nicotine derivatives were synthesized from the basic materials nicotine-N-oxide in good yields. The structures of the synthesized compounds were confirmed by spectral methods of analysis (FT-IR, 1H-NMR, and 13C-NMR). Most of the target compounds were tested for antibacterial activity against five kinds of bacteria; the tested compounds exhibited varying levels of activity against both gram-negative and gram-positive bacteria. The results of bioactivities showed that some of the target compounds exhibited good antibacterial activities against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Listeria monocytogenes, and Klebsiella pneumoniae. In addition, the broad spectrum anti-microbial action of nicotine derivatives developed in the present study may find immense applications in formulating new disinfection or decontamination strategies against widely spreading pathogens of clinical significance.
Downloads
References
2. Snyder TD. Digest of education statistics. 1993. United States Government Printing.
3. Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Develop Dynam. 1992; 194: 209-221.
4. Crooks P. N-Oxidation, N-methylation and N-conjugation reactions of nicotine. In: Proceedings of the Nicotine and Related Alkaloids. Springer, 1993: 81-109.
5. Curvall M, Vala EK. Nicotine and metabolites: analysis and levels in body fluids In: Proceedings of the Nicotine and related alkaloids. 1993: 147-179.
6. Doolittle DJ, Winegar R, Lee CK, Caldwell WS, Hayes AW, deBethizy JD. The genotoxic potential of nicotine and its major metabolites. Mutat Res Gen Toxicol. 1995; 344: 95-102.
7. Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004; 43: 619-632.
8. Ogden MW, Heavner DL, Foster TL, Maiolo KC, Cash SL, Richardson JD, et al. Personal monitoring system for measuring environmental tobacco smoke exposure. Environ Technol. 1996; 17: 239-250.
9. Lochmann H, Bazzanella A, Kropsch S, Bächmann K. Determination of tobacco alkaloids in single plant cells by capillary electrophoresis. J Chromatogr A. 2001; 917: 311-317.
10. Zettler PJ, Hemmerich N, Berman ML. Closing the regulatory gap for synthetic nicotine products. Boston College. Law School, 2018; 59: 1933.
11. Robichaud MO, Seidenberg AB, Byron MJ. Tobacco companies introduce ‘tobacco-free’nicotine pouches. Tobacco Control. 2020; 29: e145-e146.
12. Zhang H, Pang Y, Luo Y, Li X, Chen H, Han S, et al. Enantiomeric composition of nicotine in tobacco leaf, cigarette, smokeless tobacco, and e‐liquid by normal phase high‐performance liquid chromatography. Chirality. 2018; 30: 923-931.
13. Weber BT, Lothschütz C, Pan B. Preparation of racemic nicotine by reaction of ethyl nicotinate with N-vinylpyrrolidone in the presence of an alcoholate base and subsequent process steps. Google Patents 2021.
14. Chen-Sankey J, Ganz O, Seidenberg A, Choi K. Effect of a ‘tobacco-free nicotine’claim on intentions and perceptions of Puff Bar e-cigarette use among non-tobacco-using young adults. Tobacco Control. 2021: 056957.
15. Jordt S-E. Synthetic nicotine has arrived. Tobacco Control. 2021: 056626.
16. Wagner FF, Comins DL. Recent advances in the synthesis of nicotine and its derivatives. Tetrahedron. 2007; 34: 8065-8082.
17. Li L, Zou J, Xu C, You S, Li Y, Wang Q. Synthesis and anti-tobacco mosaic virus/fungicidal/insecticidal/antitumor bioactivities of natural product hemigossypol and its derivatives. J Agricult Food Chem. 2021; 69: 1224-1233.
18. Biswajit P, Albano G. Synthetic Methods for the Preparation of Conformationally Restricted Analogues of Nicotine. Molecules. 2021; 26: 7544.
19. Hellinghausen G, Lee JT, Weatherly CA, Lopez DA, Armstrong DW. Evaluation of nicotine in tobacco‐free‐nicotine commercial products. Drug Testing Anal. 2017; 9: 944-948.
20. Breining SR. Recent developments in the synthesis of nicotinic acetylcholine receptor ligands. Curr Topics Med Chem. 2004; 4: 609-629.
21. Haziza C, de La Bourdonnaye G, Skiada D, Ancerewicz J, Baker G, Picavet P, Lüdicke F. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland. Regulat Toxicol Pharmacol. 2016; 81: S139-S150.
22. Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Nicotine Psychopharmacol. 2009: 29-60.
23. Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005; 48: 4705-4745.
24. Forster M, Liu C, Duke MG, McAdam KG, Proctor CJ. An experimental method to study emissions from heated tobacco between 100-200 C. Chem Centr J. 2015; 9: 1-10.
25. Wang X, Xiao H, Wang J, Huang Z, Peng G, Xie W, et al. Synthesis and Biological Evaluation of Novel Triazine Derivatives as Positive Allosteric Modulators of α7 Nicotinic Acetylcholine Receptors. J Med Chem. 2021; 64: 12379-12396.
26. Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharmaceut Anal. 2016; 6: 71-79.
27. Davila D, Tambić T, Djokić S, Kolacny-Babić L. Disk diffusion sensitivity testing and antibacterial activity of azithromycin. Arzneimittel-Forsch. 1992; 42: 156-159.
28. Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007; 42(4): 321-324.
29. Bellotti AC. Arthropod pests. Cassava: biology, production and utilization. 2002: 209-235.
30. Hosni HM, Abdulla MM. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharmaceut. 2008; 58: 175-186.
31. Franchetti P, Pasqualini M, Petrelli R, Ricciutelli M, Vita P, Cappellacci L. Stereoselective synthesis of nicotinamide beta-riboside and nucleoside analogs. Bioorg Med Chem Lett. 2004; 14(18): 4655-4658.
32. Asif M. Antimicrobial potential of nicotinic acid derivatives against various pathogenic microbes. Eur Rev Chem Res. 2014: 10-21.

This work is licensed under a Creative Commons Attribution 4.0 International License.




