Biologically active phenolic acids produced by Aspergillus sp., an endophyte of Moringa oleifera
Abstract
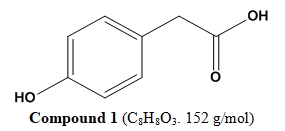
This study investigates the secondary metabolites of an endophytic Aspergillus sp. isolated from leaves of M. oleifera growing in Anambra State, South-Eastern Nigeria. Antimicrobial and antioxidant screening of the fungal extract and isolated compounds, as well as cytotoxicity assay of the extract against cisplatin-sensitive A2780 (sens) and cisplatin-resistant A2780 (cisR) ovarian cancer cell lines were carried out using standard methods. Chemical investigations of the fungal extract involving a combination of different chromato-graphic methods and spectroscopic techniques were carried out to isolate and characterize the constituents of the extract. At a concentration range of 1-4 mg/ml, the crude extract of Aspergillus sp. showed mild antimicrobial activity against Bacillus subtilis, Klebsiella pneumoniae, and Candida albicans. The fungal extract showed good antioxidant activity at 500 µg/ml, with an inhibition of 72.1%. Also, at 100 µg/ml, the extract showed excellent cytotoxic activity against A2780 (sens) and A2780 (cisR), with growth inhibitions of 105.1% and 105.5% respectively. Two known pharmacologically active phenolic compounds (p-hydroxyphenyl acetic acid and ferulic acid) were isolated from the fermentation extract of the endophytic fungus. At 250 µg/ml, ferulic acid exhibited an excellent antioxidant activity with an inhibition of 90.4%, while an inhibition of 35.4% was recorded for p-hydroxyphenyl acetic acid. Ferulic acid also showed a mild antifungal activity at 500 µg/ml against A. niger with an IZD of 2 mm. p-Hydroxyphenyl acetic acid showed no antimicrobial activity. These results further confirm the potentials of endophytic fungi associated with Nigerian plants as source of bioactive compounds with pharmaceutical or industrial applications.
Downloads
References
2. Kumar PS, Mishra D, Ghosh G, Panda CS. Medicinal uses and pharmacological properties of Moringa oleifera. Int J Phytomed. 2010; 2: 210-216.
3. Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.). J Agric Food Chem. 2003; 51(8): 2144-2155.
4. Paliwal R, Sharma V, Pracheta, Sharma S, Yadav S, Sharma S. Anti-nephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice. Biol Med. 2011; 3(2): 27-35.
5. Dahot MU. Vitamin contents of flowers and seeds of Moringa oleifera. Pak J Biochem. 1988; 21: 1-24.
6. Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J Ethnopharmacol. 2000; 69: 21-25.
7. Mehta LK, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol. 2003; 86: 191-195.
8. Caceres A, Saravia A, Rizzo S, Zabala L, Leon ED, Nave F. Pharmacologic properties of Moringa oleifera: Screening for antispasmodic, anti-inflammatory and diuretic activity. J Ethnopharmacol. 1992; 36: 233-237.
9. Morton JF. The horseradish tree, Moringa pterigosperma [Moringaceae]. A boon to arid lands? Econ Bot. 1991; 45: 318-333.
10. Gilani AH, Aftab K, Shaheen F. Antispasmodic activity of active principle from Moringa oleifera. In: Natural drugs and the digestive tract. Capasso F, Mascolo N, eds. EMSI: Rome. 1992: 60-63.
11. Gilani AH, Aftab K, Suria A, Siddiqui S, Salem R, Siddiqui BS, Faizi S. Pharmacological studies on hypotensive and spasmodic activities of pure compounds from Moringa oleifera. Phytother Res. 1994; 8: 87-91.
12. Dangi SY, Jolly CI, Narayana S. Antihypertensive activity of the total alkaloids from the leaves of Moringa oleifera. Pharm Biol. 2002; 40: 144-148.
13. Pal SK, Mukherjee PK, Saha BP. Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother Res. 1995; 9: 463-465.
14. Gilani AH, Janbaz KH, Shah BH. Quercetin exhibits hepatoprotective activity in rats. Biochem Soc Trans. 1997; 25(4): S619.
15. Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005; 1: 1-15.
16. Eilert U, Wolters B, Nadrtedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Med. 1981; 42: 55-61.
17. Bhatnagar SS, Santapau H, Desai JDH, Yellore S, Rao TNS. Biological activity of Indian medicinal plants. Part 1. Antibacterial, antitubercular and antifungal action. Indian J Med Res. 1961; 49: 799-805.
18. Caceres A, Cabrera O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991; 33: 213-216.
19. Bharali R, Tabassum J, Azad MRH. Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolizing enzymes, anti-oxidant parameters and skin papilloma genesis in mice. Asia Pacific J Cancer Prev. 2003; 4: 131-139.
20. Dhanalakshmi R, Umamaheswari S, Sugandhi P, Arvind Prasanth D. Biodiversity of the endophytic fungi isolated from Moringa oleifera of Yercaud hills. Int J Pharm Sci Res. 2013; 4(3): 1064-1068.
21. Barnabas J, Murthy SS, Jagdeesh. Antimicrobial properties of endophytic fungi isolated from Cynodon dactylon and Moringa oliefera. Int J Biol Pharma Res. 2013; 4(2): 98-104.
22. Zhao JH, Zhang YL, Wang LW, Wang JY, Zhang CL. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J Microbiol Biotechnol. 2012; 28: 2107-2112.
23. Mahdi T, Mohamed I, Yagi S. Endophytic fungal communities associated with ethnomedicinal plants from Sudan and their antimicrobial and antioxidant prospective. J Forest Prod Ind. 2014; 3(6): 248-256.
24. Eze PM, Ojimba NK, Abonyi DO, Chukwunwejim CR, Abba CC, Okoye FBC, Esimone CO. Antimicrobial activity of metabolites of an endophytic fungus isolated from the leaves of Citrus jambhiri (Rutaceae). Trop J Nat Prod Res. 2018; 2(3): 145-149.
25. Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 3rd edn. Burgress Publishing Company. New York, 1972.
26. Ainsworth GC, Sparrow FK, Sussman AS, eds. The fungi, an advanced treatise. Vol. IV(A): A taxonomic review with keys: ascomycetes and fungi imperfecti. Academic Press, New York, 1973.
27. Akpotu MO, Eze PM, Abba CC, Umeokoli BO, Nwachukwu CU, Okoye FBC, Esimone CO. Antimicrobial activities of secondary metabolites of endophytic fungi isolated from Catharanthus roseus. J Health Sci. 2017; 7(1): 15-22.
28. Mueller H, Kassack MU, Wiese M. Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J Biomol Screen. 2004; 9: 506-515.
29. Engelke LH, Hamacher A, Proksch P, Kassack MU. Ellagic acid and resveratrol prevent the development of cisplatin resistance in the epithelial ovarian cancer cell line A2780. J Cancer. 2016; 7(4): 353-363.
30. Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. Antioxidant activity in vitro of selenium-contained protein from the se-enriched Bifodobacterium animalis 01. Anaerobe. 2010; 16: 380-386.
31. Ohtani K, Fujioka S, Kawano T, Shimada A, Kimura Y. Nematicidal activities of 4-hydroxyphenylacetic acid and oidiolactone D produced by the fungus Oidiodendron sp. Z Naturforsch C. 2011; 66(1-2): 31-34.
32. Abe H, Uchiyama M, Sato R. Isolation of phenylacetic acid and its p-hydroxy derivative as auxin-like substances from Undaria pinnatifida. Agr Biol Chern. 1974; 38 (4): 897-898.
33. Sajjadi SE, Shokoohinia Y, Moayedi N. Isolation and identification of ferulic acid from aerial parts of Kelussia odoratissima Mozaff. Jundishapur J Nat Pharm Prod. 2012; 7(4): 159-162.
34. El-gizawy HA, Hussein MA. Isolation, structure elucidation of ferulic and coumaric acids from Fortunella japonica Swingle leaves and their structure antioxidant activity relationship. Free Rad Antiox. 2017; 7(1): 23-30.
35. Gao Y, Guo C, Zhang Q, Zhou W, Wang CCC, Gao J. Asperaculanes A and B, two sesquiterpenoids from the fungus Aspergillus aculeatus. Molecules. 2015; 20: 325-334.
36. Bai Z, Lin X, Wang J, Zhou X, Liu J, Yang B, et al. New meroterpenoids from the endophytic fungus Aspergillus flavipes AIL8 derived from the mangrove plant Acanthus ilicifolius. Mar Drugs. 2015; 13: 237-248.
37. Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014; 78: 141-173.
38. Ge HM, Yu ZG, Zhang J, Wu JH, Tan RX. Bioactive alkaloids from endophytic Aspergillus fumigatus. J Nat Prod. 2009; 72(4): 753-755.
39. Carbungco ES, Pedroche NB, Panes VA, de la Cruz TE. Identification and characterization of endophytic fungi associated with the leaves of Moringa oleifera Lam. Acta Hortic. 2017; 1158: 373-380.
40. Rajeswari S, Umamaheswari S, Prasanth DA, Rajamanikandan KCP. Study of endophytic fungal community of Moringa oleifera from Omalur Region - Salem. Int J Pharm Sci Res. 2014; 5(11): 4887-4892.
41. Budhiraja A, Nepali K, Sapra S, Gupta S, Kumar S, Dhar KL. Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn. Med Chem Res. 2012; 22(1): 323-329.
42. Shaaban M, Hamdi Nasr H, Hassan AZ, Asker MS. Bioactive secondary metabolities from endophytic Aspergillus fumigatus: Structural elucidation and bioactivity studies. Rev Latinoam Quím. 2013; 41(1): 50-60.
43. You Y, Kwak TW, Kang S, Lee M, Kim J. Aspergillus clavatus Y2H0002 as a new endophytic fungal strain producing gibberellins isolated from Nymphoides peltata in fresh water. Mycobiol. 2015; 43(1): 87-91.
44. Danagoudar A, Joshi CG, Kumar SR, Poyya J, Nivya T, Hulikere MM, Appaiah KAA. Molecular profiling and antioxidant as well as anti-bacterial potential of polyphenol producing endophytic fungus - Aspergillus austroafricanus CGJ-B3. Mycology. 2017; 8(1): 28-38.
45. Wang H, Eze PM, Hӧfert S, Janiak C, Hartmann R, Okoye FBC, et al. Substituted L-tryptophan-L-phenyllactic acid conjugates produced by an endophytic fungus Aspergillus aculeatus using an OSMAC approach. RSC Adv. 2018; 8: 7863-7872.
46. Abba CC, Nduka I, Eze PM, Ujam TN, Abonyi DO, Okoye FBC. Antimicrobial activity of secondary metabolites of endophytic Aspergillus species isolated from Loranthus micranthus. Afr J Pharm Res Dev. 2016; 8(2): 136-140.
47. Papadopoulos G, Boskou D. Antioxidant effect of natural phenols on olive oil. J Am Oil Chem Soc. 1991; 68(9): 669.
48. Nardini M, Ghiselli A. Determination of free and bound phenolic acids in beer. Food Chem. 2004; 84(1): 137-114.
49. Chapla VM, Zeraik ML, Leptokarydis IH, Silva GH, Bolzani VS, Young MCM, et al. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules. 2014; 19: 19243-19252.
50. Zuo WJ, Jin PF, Dong WH, Dai HF, Mei WL. Metabolites from the endophytic fungus HP-1 of Chinese eaglewood. Chin J Nat Med. 2014; 12(2): 151-153.
51. Kumar N, Pruthi V. Structural elucidation and molecular docking of ferulic acid from Parthenium hysterophorus possessing COX-2 inhibition activity. 3 Biotech. 2015; 5(4): 541-551.
52. Rosazza JPN, Huang Z, Dostal L, Volm T, Rousseau B. Review: biocatalytic transformations of ferulic acid: an abundant aromatic natural product. J Ind Microbiol. 1995; 15: 457-471.
53. Cheng M, Wu M, Chen J, Cheng Y, Hsieh M, Hsieh S, et al. Secondary metabolites from the endophytic fungus Annulohypoxylon stygium BCRC 34024. Chem Nat Comp. 2014; 50(2): 237-241.
54. Schmidt CG, Gonçalves LM, Prietto L, Hackbart HS, Furlong EB. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014; 146: 371-377.
55. Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992; 3: 435-513.
56. Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002; 50: 2161-2169.
57. Balasubashini MS, Rukkumani R, Viswanathan P, Menon VP. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother Res. 2004; 18: 310-314.
58. Nomura E, Kashiwada A, Hosoda A, Nakamura K, Morishita H, Tsuno T, Taniguchi H. Synthesis of amide compounds of ferulic acid and their stimulatory effects on insulin secretion in vitro. Bioorg Med Chem. 2003; 11: 3807-3813.
59. Suzuki A, Kagawa D, Fujii A, Ochiai R, Tokimitsu I, Saito I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am J Hypertens. 2002; 15: 351-358.
60. Ohsaki AY, Shirakawa H, Koseki T, Komai M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J Agric Food Chem. 2008; 56: 2825-2830.
61. Mori H, Kawabata K, Yoshimi N, Tanaka T, Murakami T, Okada T, Murai H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999; 19: 3775-3783.
62. Hudson EA, Dinh PA, Kokubun T, Simmonds MSJ, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomark Prev. 2000; 9(11): 1163-1170.
63. Ou S, Kwok KC. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric. 2004; 84: 1261-1269.
64. Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007; 40(2): 92-100.
65. Okoye FBC, Nworu CS, Debbab A, Esimone CO, Proksch P. Two new cytochalasins from an endophytic fungus, KL-1.1 isolated from Psidium guajava leaves. Phytochem Lett. 2015; 14: 51-55.
66. Ebada SS, Peter Eze, Okoye FBC, Esimone CO, Proksch P. The fungal endophyte Nigrospora oryzae produces quercetin monoglycosides previously known only from plants. Chem Select. 2016; 1: 2767-2771.
67. Okezie UM, Eze PM, Okoye FBC, Ikegbunam MN, Ugwu MC, Esimone CO. Biologically active metabolites of an endophytic fungus isolated from Vernonia amygdalina. Afr J Pharm Res Dev. 2017; 9(1): 24-29.
68. Abba CC, Eze PM, Abonyi DO, Nwachukwu CU, Proksch P, Okoye FBC, Eboka CJ. Phenolic compounds from endophytic Pseudofusicoccum sp. isolated from Annona muricata. Trop J Nat Prod Res. 2018; 2(7): 332-337.
69. Nnanna JC, Eze PM, Anyanwu OO, Ujam TN, Ikegbunam MN, Okoye FBC, Esimone CO. Screening of metabolites of endophytic fungi isolated from leaves of Azadirachta indica for antimicrobial and cytotoxic activities. Pharm Chem J. 2018; 5(3): 20-27.
70. Nwachukwu CU, Ngwoke KG, Eze PM, Eboka CJ, Okoye FBC. Secondary metabolites from Curvularia sp, an endophytic fungus isolated from the leaves of Picralima nitida Durand and Hook (Apocynaceae). Trop J Nat Prod Res. 2018; 2(5): 209-213.
71. Akpotu MO, Eze PM, Abba CC, Nwachukwu CU, Okoye FBC, Esimone CO. Metabolites of endophytic fungi isolated from Euphorbia hirta growing in Southern Nigeria. Chem Sci Rev Lett. 2017; 6(21): 12-19.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




